A Neutral Atom Contains 12 Neutrons and 11 Electrons
A sodium Na atom contains 11 protons 12 neutrons and 11 electrons. Virtual Families 3 Free Download Full Version Virtual Families 3 Mod Apk 1.
A neutral atom with 11 electrons would by definition have to have 11 protons.

. A sodium atom contains 11 protons 12 neutrons and 11 electrons. Naturally occurring chlorine consists primarily of two isotopes. The kind of energy.
Sodium has 11 protons 12 neutrons and 11 electrons. How may electrons in the neutral atom. If an atom loses or gains electrons it is called an ion.
This atom would contain _____ protons _____ neutrons and _____ electrons. One atom the sodium has 11 protons 11 electronsand 12 neutrons what is its atomic number and mass. Aluminum has 13 protons 14 neutrons and 13 electrons.
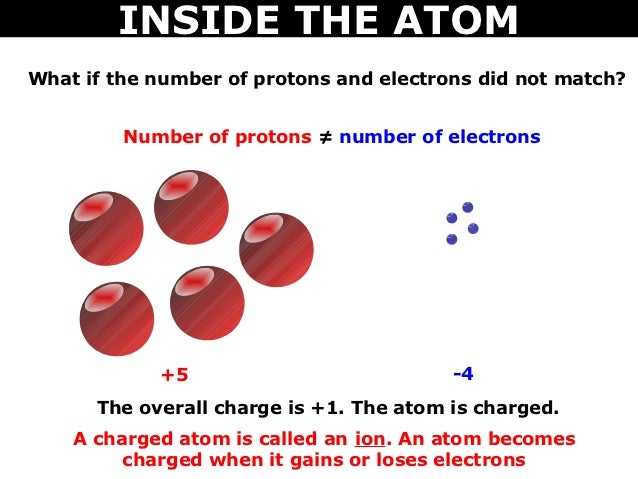
Thanks for asking the question. Neon has 10 protons 10 neutrons and 10 electrons. Correct answer - A neutral atom contains 12 neutrons and 11 electrons.
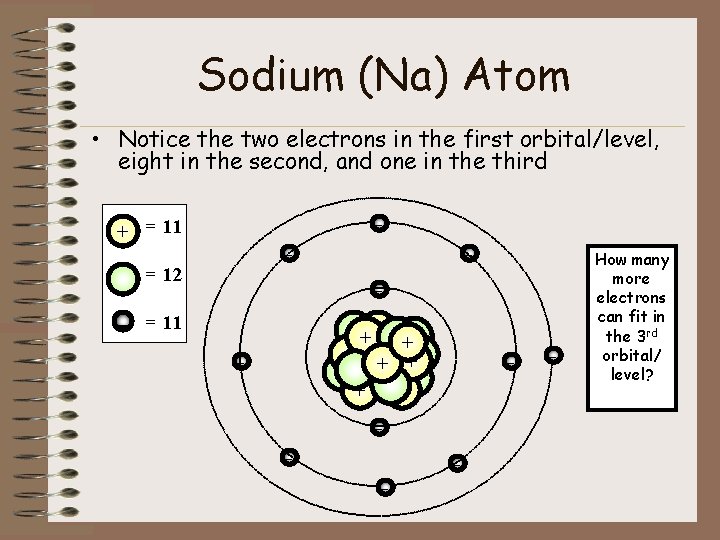
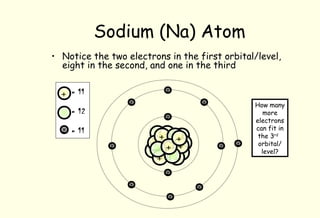
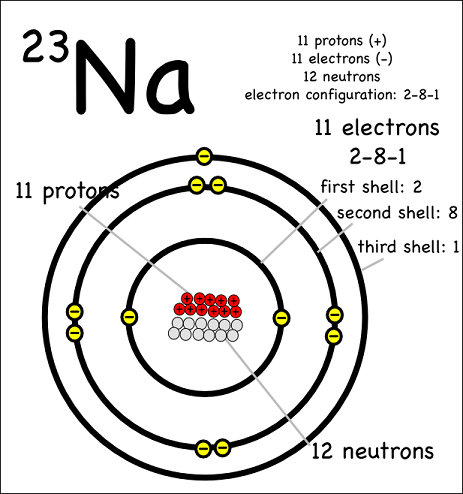
Mass number is the total of the protons and neutrons together thus sodium has a mass. With 11 protons and 12 neutrons its molecular weight is 23 -- the most common atomic mass of Na. Thats why Sodiums neutral mass is 23.
We know that the atomic number of sodium is 11. To make the sodium ion NA it must loose a_____. A neutral atom has 12 electrons and 13 neutrons.
A neutral atom contains 12 neutrons and 11 electrons The number of protons in from PHYSICS 1 at Western University. Hence the correct option is A Solve any question of Structure of Atom with-. How many electrons does the atom have in its neutral state.
In order to make the sodium ion Na what must occur to the sodium atom. You need to understand what is inside those circle which you use to. In this case the Protons have an average of 2 units of charge.
A neutral atom contains 11 protons 12 neutrons and 11 electrons. In this case we are talking about Sodium. When some subatomic particles split from each other energy is released.
Answer 1 of 2. A magnesium atom has an atomic number of 12 an atomic mass of 25 and a 2 charge. An atom with 11 electrons and presumably 11 protons has the atomic number of 11.
This tells us that sodium has 11 protons and because it is neutral it has 11 electrons. A It must gain one electron. It would be worth your while to review these definitions.
A mass number of 23 means 23 11 this atom will have 12 neutrons. If there are 11 protons THERE MUST be 11 electrons orbiting around the atom because the element here is NEUTRAL. The term we would use for this element is 23N a.
The mass number of an element tells us the number of protons AND neutrons in an atom the two particles that have a measurable mass. If it is a 23N a nuclide there must be 12 neutrons in the nucleus. Atomic mass Noof protons No.
Magnesium has 12 protons 12 neutrons and 12 electrons. Correct option is A The atomic mass of an atom is the sum of protons and neutrons in an atom. This atom will have 11 electrons.
Silicon has 14 protons 14 neutrons and 14 electrons. But the number of nucular protons ie. There are only 12 Baryons in any atom in total and half of those are Neutrons.
The electrical charge of the nucleus of an atom is 12. The answer is obviously 12 but the model is naïve. Z UNEQUIVOCALLY identifies the atom.
Phosphorous has 15 protons 16 neutrons and 15 electrons. Since this atom is neutral the positive protons must be equal to the negative electrons. A single sodium atom has 11 protons 11 electrons and 12 neutrons.
An atomic number of 11 means this atom will have 11 protons. Atomic Number 11and number of neutrons is 12 and number of protons is 11. A neutral atom contains 12 neutrons and 11 electrons.
How many neutrons and also protons does a neutral atom the sodium v an atom mass number of 23 have. What is the atom. Z 11 for sodium.
Determine the number of protons electrons and neutrons for an atom of each isotope. The sum of neutrons and protons the massive nuclear particles gives the mass number with which we often label the elemental symbol as a left hand superscript. For any neutral atom the number of protons is the same as the number of electrons.
If it is a sodium atom then Z 11 by definition. The number of protons in this atom is. The number of protons in this atom is.
A sodium atom contains 11 protons so its atomic number is 11. The number of neutrons in the nucleus of an atom can be determined by Subtracting the atomic number from the mass number of the atom A neutral. The atom is of- a potassium b sodium c lithium d magnesium.
What is its mass number. An atom contains 11 protons and 12 neutrons.
An Atom Consists Of 12 Neutrons And 11 Protons What Is The Atomic Number And The Mass Quora

Structure Of The Atom Unit Middle School Science Classroom Reading Comprehension Activities Comprehension Activities

3 4 Atomic Mass And Atomic Number Chemistry Libretexts Periodic Table Mass Number Atomic Number

Handouts And How To S Melody Weintraub
The Building Blocks Of Matter Atoms Atoms Smallest

A Neutral Atom Contains 12 Neutrons And 11 Electrons The Number Of Protons In This Atom Is 1 1 3 Brainly Com

Consider A Wooden Chair And A Balloon Ppt Download

Forces And Motion Worksheet Luxury Intro To Energy Worksheet Or Homework Energy Forces Chessmuseum Template Lib Chemistry Worksheets Atomic Theory Worksheets

Pin By Vo Thanh On Facultad Quimica Teaching Chemistry Chemistry Classroom Chemistry Lessons

Virtually Everything That Is Is Made Up Of Atoms Chapter 12 Page 361 Balloon And Sweeter Animation Ppt Download

History Of The Atom And Basic Knowledge About Subatomic Particles Atom Atomic Structure Knowledge

Elements And Atoms Ppt Download

Solved If An Atom Of An Element Has 11 Protons And 12 Neutrons In I

A Battery Operated Car Utilizes A 120 0 V Battery With Negligible Internal Resistance In 2022 Battery Operated Resistance Battery

Comments
Post a Comment